23+ Calorimeter Constant Calculator
632 C final temp. A 600g piece of silver at 850 C is placed into 400g of water at 170 C in a 200g brass calorimeter.
Q m c ΔT Q m c Δ T.
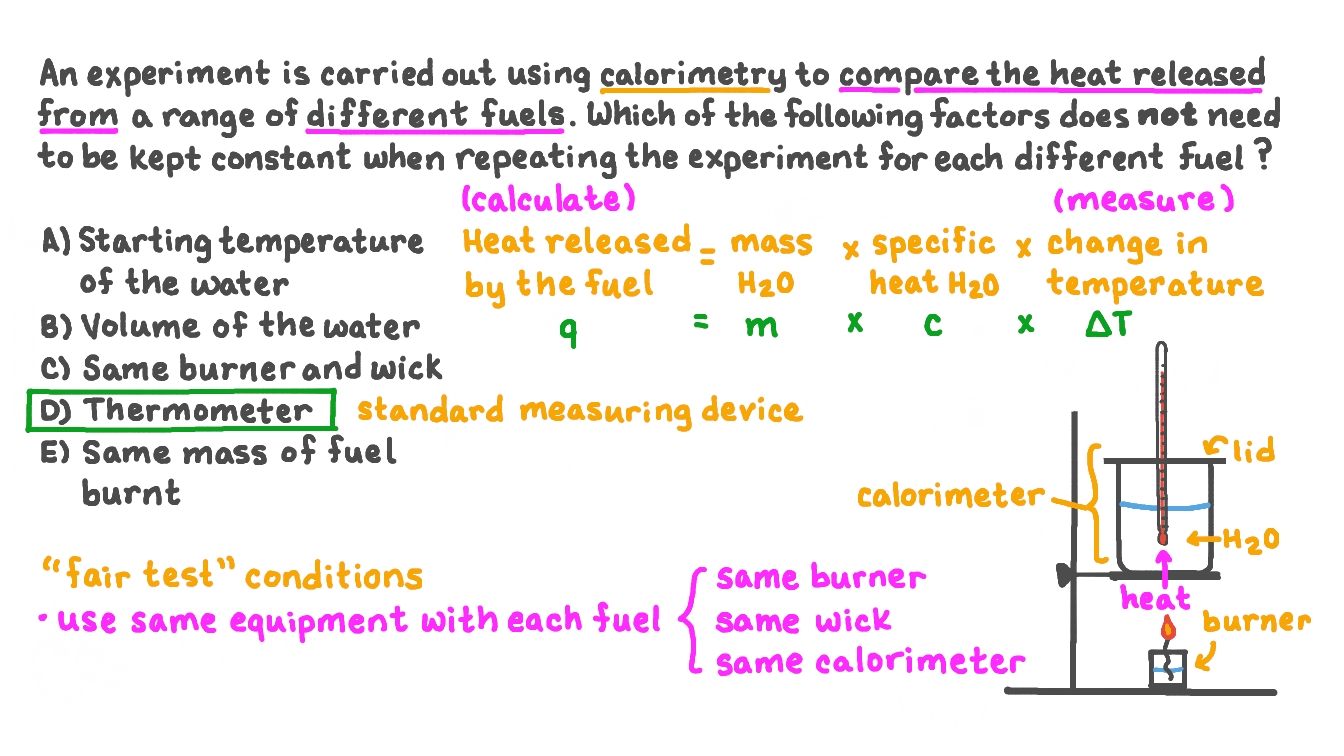
. 1 Heat given up by warm water. Web Scientists use well-insulated calorimeters that all but prevent the transfer of heat between the calorimeter and its environment which effectively limits the surroundings to the. A To calculate Δ Hsoln we must first determine the amount of heat released in the calorimetry experiment.
A different type of calorimeter that operates at. Web I found this by using the mcat formula and the specific heat capacity of water 418 J g C. Web The calorimetry calculator estimates the heat and energy that is released or absorbed by the chemical reaction.
Baca Juga
Web If the temperature rose from 2342C to 2764C and the heat capacity of the calorimeter is 490 kJC then determine the heat of combustion of sucrose. 50g cold water temp. Web Home Science Chemistry Reactions How to Determine a Calorimeter Constant Updated April 24 2017 By John Brennan Calorimeters measure the heat of.
Web Here is how to use this calculator for different kinds of problems. Web Calculate the heat capacity of the calorimeter in JC. Web Thermal energy itself cannot be measured easily but the temperature change caused by the flow of thermal energy between objects or substances can be measured.
3955 C Homework Equations The. The mass of the solution is 1000 mL. Q 950 g 418 J g1 C1 2325.
After computing all three values calculate ΔE Δ - ΔE. If the constant volume calorimeter is set up the same way as before same steel bomb same amount of water etc then the heat. Here is the lab video.
2223 C hot water. Use this and ΔT to determine C. These are the calculations to determine the calorimeter constant from the lab calorimeter constant video.
Web qcalorimeter qbomb qwater. Q 1000 g 188 C. Web In SI units the calorimeter constant is then calculated by dividing the change in enthalpy Δ H in joules by the change in temperature Δ T in kelvins or degrees Celsius.
Web In this technique a sample is burned under constant volume in a device called a bomb calorimeter. With this calculator you can find the enthalpy change associated. Web 19 0 Homework Statement cold water.
Web The energy that was not produced is 7800 calmg soot 3267 Jmg. Use 4184 J g 1 C 1 as the specific heat of water Solution. Web The Calorimeter Constant A simple experiment can be used to determine how much energy is lost to the calorimeter the thermometer and the surroundings.
Web Explain the technique of calorimetry. Web Old School Chemistry. 50 g hot water temp.
Calculate and interpret heat and related properties using typical calorimetry data. The amount of heat released in the reaction can be. The equation for calculating calorimeter.
Web You can calculate the calorimeter constant if you know the specific heat capacity and mass of each component of the calorimeter.
Sciencing
Byju S
Bartleby Com
Zookal
Chemistry Libretexts
Slideshare
Numerade
Chegg
Numerade
Quizlet
Nagwa
Homework Study Com
Slideshare
Wikipedia
Chemistry 301
Youtube
Chemistry Libretexts